
Hideko Kanazawa
Keio University
Faculty of Pharmacy
Professor
In recent years, development of new drugs by pharmaceutical companies has become difficult, with many candidates being eliminated at late stages of development, and the conditions for approval having become increasingly rigorous. In the context of changes in various aspects of the current situation, including revision of approved drug prices, life-cycle management, involving maximization of the value of existing products, extension of product life spans, and various measures to increase profits over as long a period as possible, has become an important focus of business strategy. In this context, one opinion is that the limit for development of single-component drugs, such as have been in use previously, is being reached. An alternative is combination drugs, in which two or more types of pharmacologically active component are combined in a single formulation, and, since 2006, when the first antihypertensive combination drug was launched, the number of such drugs in the ethical pharmaceutical market has increased rapidly, as an aspect of life-cycle management, and as a response to the prevalence of generic agents. In this manner, attention is being given to combination drugs as an approach to new drug development, and it is hoped that combination drugs containing drugs with different mechanisms of action will become therapeutic agents with which breakthroughs will be made in development areas that have been focused on monotherapy. Most such drugs are therapeutic agents for chronic diseases such as hypertension, diabetes, and asthma, and, on the basis of the accepted concept, although the overwhelming majority are combinations of two or more approved drugs, it is certainly possible that in future highly revolutionary formulations that combine approved and unapproved, or unapproved and unapproved, drugs will be developed.
Combination drugs containing existing drugs offer various advantages, including decrease in the number of drugs that need be taken, improved patients' adherence to the regimen, increased convenience, and suppression of drug cost increases. Disadvantages that have been put forward include difficulties in identifying the causes of adverse effects, and inability to adjust the dose, but these are outweighed by the usefulness of the drugs, and the number of prescriptions is therefore increasing. On the other hand, as combination drugs have only recently become commercially available, the high-performance liquid chromatography (HPLC) conditions needed for quantification of each of the components of multi-component drugs, and for monitoring formulation stability, and in vivo concentrations, have not been established. In addition, sufficient data have not yet been obtained on the differences between components in combination tablets when these are administered in accordance with different regimens, and on whether or not there are changes in the components when combination drugs are administered together with other drugs, so the current situation is that, if essential information is absent, there are difficulties involved even in assessing the appropriateness of drug combinations that have been prescribed in the clinical environment. Therefore, requirements for information relating to the appropriateness and stability of use of drug combinations are increasing. In the Japanese Pharmacopoeia (JP), identification tests and quantitative assay methods are stipulated for each individual drug included, but no such tests are stipulated for combination drugs, with two or more components. In the 16th edition of the JP, among the general test methods, the stipulations in the section about chromatography have been revised. In the case of identification tests carried out using HPLC, it is suggested that in some situations a photodiode array (PDA) detector should be used, and the reasoning in this respect is given as follows:
If the detector used simultaneously collects information relating to the molecular structures of the components tested, a highly specific identification test can be carried out, on the basis of compatibility of the information relating to molecular structures, as well as compatibility of retention times.
In the present research, methods were investigated for rapid, simultaneous quantification and identification of the active components of combination drugs using ultra-high-performance liquid chromatography (UHPLC) in combination with PDA detectors.
In recent years, in connection with major changes in lifestyles, and the aging of society, the numbers of diabetic and pre-diabetic patients have shown a tendency to increase. Diabetes is classified as types 1 and 2. Type-1 diabetes involves insufficient insulin activity, which is primarily caused by degradation and loss of β-cells in the pancreatic islets of Langerhans, whereas type-2 diabetes involves decreased insulin secretion, and insulin resistance, which are due to aging, and environmental factors such as overeating, lack of exercise, obesity, and stress. Approximately 90% of Japanese diabetes patients have the type-2 diabetes, and diagnosis and treatment of type-2 diabetes is expected to be increasingly important. Type-2 diabetes is treated with drug therapy if the blood glucose levels cannot be controlled by exercise and dietary therapy, but the drugs used sometimes have the adverse effect of serious hypoglycemia. As drugs that do not readily cause hypoglycemia, glucagon-like peptide 1 (GLP-1) -receptor agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors have been developed recently. GLP-1-receptor agonists are self-injected agents, whereas DPP-4-inhibitors are orally administered, and therefore superior in terms of convenience, so most attention has been given to the latter1-6). However, when DPP-4-inhibitors are administered together with other antidiabetic drugs, the combinatorial effects of blood glucose control increase the risk of hypoglycemia, and care is therefore needed in this respect. In order to provide antidiabetic drug therapy that is appropriate for patients, drugs are often administered concomitantly.
In recent years, there have been commercial launches of orally administered combination tablets for antidiabetic therapy, containing DPP-4-inhibitors as active components. With respect to simultaneous analysis by HPLC of two types of active component in combination tablets, there have already been reports about some combinations7-9). However, there have been no reports of simultaneous, multi-component analysis concerned primarily with the active components included in combination tablets that are commercially available in Japan. In the present research, attempts were made to carry out integrated, simultaneous analysis of oral antidiabetic drugs, by means of HPLC and UHPLC, focusing on DPP-4-inhibitors that are not yet included in the JP, and other new oral antidiabetic drugs, and the findings were applied to SONIAS® and LIOVEL®, which are combination tablets manufactured by Takeda Pharmaceutical Co., Ltd.
The molecular structures of the active components of oral antidiabetic drugs are shown in Fig. 1.
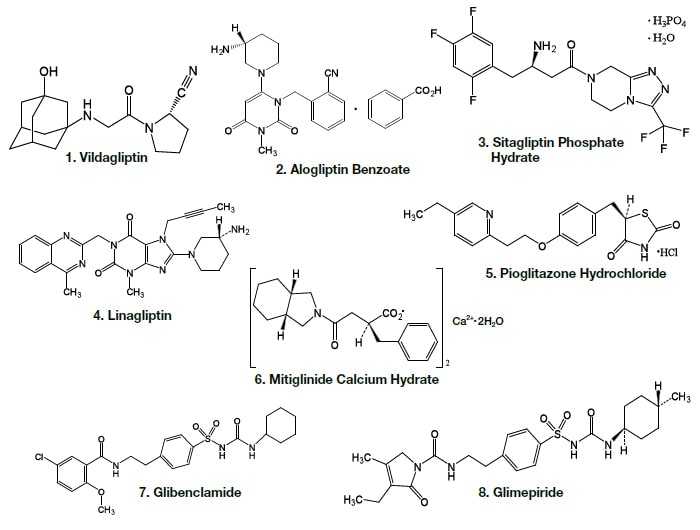
Fig. 1 Molecular structures of active components of oral antidiabetic drugs
The HPLC system used consisted of a pump (HITACHI L-7100), a detector (HITACHI L-7405), a column oven (SSC-2120), an autosampler (HITACHI L-2200), data system software (D-2000 Elite), and a column (HITACHI LaChrom C18, 5 µm) with an internal diameter of 4.6 mm and a length of 150 mm. UHPLC system consisted of a pump (HITACHI L-2160U), a PDA detector (HITACHI L-2455U), a column oven (HITACHI L-2300), an autosampler (HITACHI L-2200U), data system software (HITACHI EZChrom Elite), and a column (HITACHI LaChromUltra C18, 2 µm) with an internal diameter of 2.0 mm and a length of 50 mm.
Each of the combination tablets was pulverized, dissolved in methanol, subjected to ultrasonication for 30 minutes, and then centrifuged, after which the supernatant was collected. The standard material was dissolved in methanol, and filtered using a 0.2 µm membrane filter, to prepare a 1 mg/mL solution. Each of the solutions obtained was diluted with methanol to prepare the sample solutions. The mixed solutions were prepared by mixing the relevant 1 mg/mL solutions to each sample solution concentration.
Analysis of the following eight active components of oral antidiabetic drugs was carried out by HPLC and UHPLC: vildagliptin, alogliptin benzoate, sitagliptin hydrochloride, linagliptin, pioglitazone hydrochloride, mitiglinide calcium hydrate, glibenclamide, and glimepiride. The chromatograms obtained by HPLC are shown in Fig. 2, and those obtained using a HITACHI LaChromUltra are shown in Fig. 3. With both HPLC and UHPLC analysis, alogliptin benzoate showed two peaks, and it was confirmed that the first peak was alogliptin, and the second was benzoate. On the other hand, the time needed for analysis by UHPLC was approximately 20% that needed for HPLC. The use of UHPLC enables reduction in the analysis time, the sample volumes needed for measurement, and the volume of mobile phase used, thus enabling analysis to be carried out more efficiently. The outcome of investigation of the application to combination tablets using the LaChromUltra was that, irrespective of the formulation, two types of active component in oral antidiabetic drugs were separated effectively within a short time. The chromatograms for SONIAS® combination tablets, which contain pioglitazone and glimepiride, and LIOVEL® combination tablets, which contain pioglitazone and alogliptin, are shown in Fig. 4. Both of these combination tablets are drugs with which there is the potential to alleviate both of the pathological states involved in type-2 diabetes, insulin resistance and insufficient insulin secretion, with administration of a single tablet, and they are also expected to have long-term protective effects on pancreatic β-cells. Each of the drugs is administered once per day, and it is therefore also expected that patients' adherence will be improved by the use of combination tablets.
The construction of a simultaneous, multi-component analysis method has the potential for application to analysis of increasing numbers of combination tablets in future, and to drug monitoring in order to reduce adverse effects in the clinical environment. In addition, attention has been given to DPP-4-inhibitors as drugs that do not readily induce hypoglycemia, and it is considered that such analysis will be useful for analysis of oral antidiabetic drugs, especially DPP-4-inhibitors10).
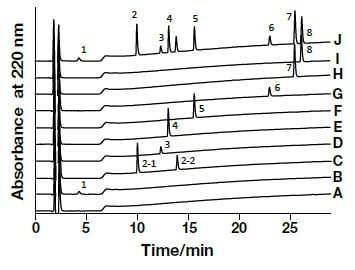
Fig. 2 Chromatograms of oral antidiabetic drugs obtained by general-use HPLC
CH3OH (A), Vildagliptin (1,B), Alogliptin Benzoate (2,C), Sitagliptin Phosphate Hydrate (3,D), Linagliptin (4,E), Pioglitazone Hydrochloride (5,F), Mitiglinide Calcium Hydrate (6,G), Glibenclamide (7,H), Glimepiride (8,I), mixed solution (J)Column, HITACHI LaChrom C18, 5 µm (4.6 mm I.D.×150 mm); eluent, a) 10 mM HCOONH4/CH3CN/HCOOH = 900/100/1, b) CH3CN/HCOOH = 1000/1; gradient, a/b = 0 min 100/0→2 min 100/0→27 min 30/70; flow rate, 1.0 mL/min; column temp., 30°C; detection, UV220 nm; pressure, 10.6 MPa; inj.vol., 10 µL
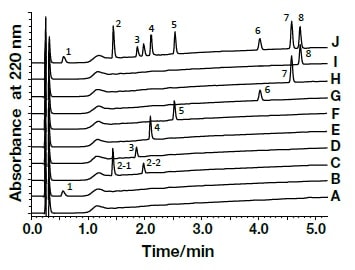
Fig. 3 Chromatograms of oral antidiabetic drugs obtained by ultra-high-speed HPLC
CH3OH (A), Vildagliptin (1,B), Alogliptin Benzoate (2,C), Sitagliptin Phosphate Hydrate (3,D), Linagliptin (4,E), Pioglitazone Hydrochloride (5,F), Mitiglinide Calcium Hydrate (6,G), Glibenclamide (7,H), Glimepiride (8,I), mixed solution (J)Column, HITACHI LaChromUltra C18, 2 µm (2.0 mm I.D.×50 mm); eluent, a) 10 mM HCOONH4/CH3CN/HCOOH = 900/100/1, b) CH3CN/HCOOH = 1000/1; gradient, a/b = 0 min 100/0→5 min 40/60; flow rate, 0.6 mL/min; column temp., 30°C; detection, UV220 nm; pressure, 33.0 MPa; inj.vol., 1 µL
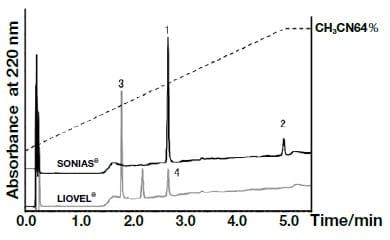
Fig. 4 Chromatograms of the combination tablets SONIAS®and LIOVEL®
Antihypertensive drugs include calcium-channel-blockers (dihydropyridine and benzothiazepine drugs), angiotensin-II-receptor-blockers (ARBs), angiotensin-converting-enzyme-inhibitors, diuretics (thiazides, and loop diuretics), β-blockers, α-blockers, and combination drugs, which combine diuretics and ARBs, or calcium-channel-blockers and ARBs. Hypertension is a disease with multiple causes, so, in order to meet the target blood pressure, combination therapy involving multiple antihypertensive drugs with different mechanisms of action is useful. It is therefore expected that administration of combination drugs containing multiple therapeutic agents in a single formulation will reduce the burden on patients administered multiple drugs, and will contribute to improvements in therapeutic efficacy. In Japan, with its aging population, it is no exaggeration to say that preventing hypertension and other lifestyle-related diseases is a matter of national importance.
ARBs have various effects in addition to antihypertensive activity. The following seven ARBs are currently in clinical use in Japan: losartan, valsartan, candesartan, telmisartan, olmesartan, irbesartan, and azilsartan. Recent research comparing different ARBs has suggested differences in antihypertensive and organoprotective activities.
Olmesartan, a component of REZALTAS® combination tablets (Daiichi Sankyo Co., Ltd.), is converted rapidly to the active form. It shows strong, noncompetitive inhibition, and peak activity is reached more rapidly than with candesartan. In common with valsartan and candesartan, olmesartan has hydroxyl groups as well as carboxyl groups, and both of these groups are thought to bind to the angiotensin-II-receptor.
The analysis methods for olmesartan medoxomil and azelnidipine, which are components of REZALTAS® combination tablets, are not stipulated in the JP.
In the present research, rapid and efficient analysis methods using UHPLC, with PDA detectors, were investigated with respect to the components of REZALTAS® combination tablets. Simultaneous analysis conditions for the HD component of REZALTAS® combination tablets (olmesartan medoxomil + azelnidipine) was investigated, and the resulting chromatogram is shown in Fig. 5. Peaks were found after 14.18 and 7.50 minutes for azelnidipine and the internal standard substance, respectively. Peaks were also found after 1.85 and 4.26 minutes, and these were thought to be olmesartan medoxomil and a component from which this was derived. Under similar analysis conditions to those used for the Rezaltas® HD component (olmesartan medoxomil + azelnidipine), the following single-component formulations were analyzed, and the results are shown in Fig. 6: Olmetec® tablets 20 mg (Daiichi Sankyo Co., Ltd.), which contain only olmesartan medoxomil; and Calblock® tablets 16 mg (Daiichi Sankyo Co., Ltd.), which contain only azelnidipine. Peak 2 in the chromatogram is considered to be olmesartan medoxomil, but the Rezaltas® HD component has peaks 1 and 2 of approximately the same height, and peak 1, albeit small, was even found with Olmetec® tablets.
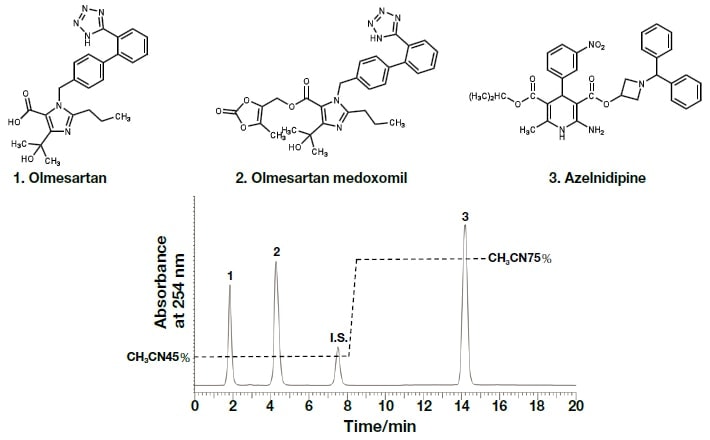
Fig. 5 Chromatogram and molecular structure of the Rezaltas® HD component (olmesartan medoxomil + azelnidipine)
Column: HITACHI LaChromUltra C18, 5 µm (4.6 mm I.D.×150 mm), eluent: a) KH2PO4(41→10000): CH3CN = 55:45, b) KH2PO4(41→10000): CH3CN = 25:75, gradient: a/b = 0 min;55/45→8.0 min;55/45→8.1 min;25/75→15 min;25/75, flow rate:1.0 mL /min, column temp.: 25°C, detection:254 nm, pressure:6.2 MPa, inj.vol.: 20 µL
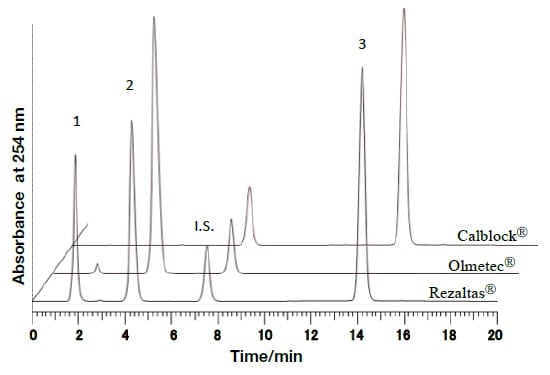
Fig. 6 Comparative investigation of chromatograms of different formulations under the analysis conditions used for the Rezaltas® HD component (olmesartan medoxomil+azelnidipine)
In order to identify the above two components, the spectra were compared using UHPLC with a PDA detector (Fig. 7). The analysis duration was excellent, being less than 2 minutes. The spectrum reported by Murakami et al.11) and that detected using the PDA detector, as shown in Fig. 8, were consistent, and it can therefore be concluded that in both cases peak 1 was olmesartan, and peak 2 was olmesartan medoxomil.
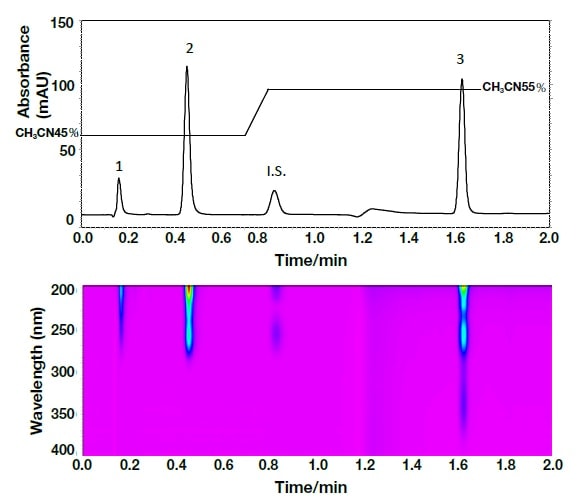
Fig. 7 Chromatogram and contour plot for the Rezaltas® HD component (olmesartan medoxomil+azelnidipine) obtained by ultra-high-speed HPLC
Column: HITACHI LaChromUltra C18, 2 µm (2.0 mm I.D.×50 mm), eluent: a) KH2PO4(41→10000): CH3CN = 55:45, b) KH2PO4(41→10000): CH3CN = 25:75, gradient: a/b = 0 min; 100/0→0.7 min; 100/0→ 0.8 min; 0/100→1.7 min; 0/100, flow rate: 0.8 mL/min, column temp.: 30°C, detection: 254 nm, 200-400 nm (PDA) pressure: 40-49 MPa, inj.vol.: 1 µL
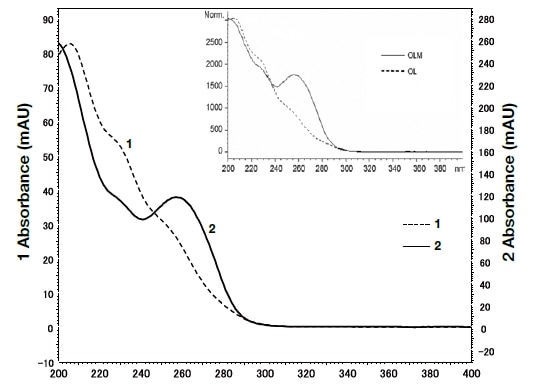
Fig. 8 Spectra for peaks 1 and 2 shown by ultra-high-speed HPLC with a PDA detector
The graph within the inset box shows the published spectra for olmesartan medoxomil and azelnidipine11).
As detailed above, combination antihypertensive drugs have two components that differ markedly in terms of solubility and polarity. Therefore, if rapid, simultaneous analysis is carried out in connection with dissolution and stability tests of combination drug components, there is the potential for the measurement time with UHPLC to be markedly reduced in comparison with the HPLC in general use. It is hoped that, with use of UHPLC together with a PDA detector, the identification tests and component quantitative assays stipulated in the JP can be carried out as a single analysis12).
References
See more