Masahito Matt Ito*1, Ikuko Narimatsu*2, Katsutoshi Shimizu*2
In October 2020, the Division of Liquid Chromatography in the Japan Society for Analytical Chemistry officially recognized Hitachi’s Model 835 high-speed amino acid analyzer—an instrument equipped with advanced capabilities that significantly influenced the development of high performance liquid chromatography (HPLC)—as the third in a series of instruments representing historic milestones in the progress of science, that is the Science Heritage. The Model 835 (Figure 1), an archetypical amino acid analyzer in Japan, was recognized for the prominent role it played in the evolution of the field, with the following novel technologies and new capabilities noted in particular:
In this article we will revisit the above points in detail.

Fig. 1 Hitachi’s Model 835 high-speed amino acid analyzer (1977). Whereas previous amino acid analyzers had been tall, the Model 835 was able to achieve a shorter footprint—for a reason.
The conceptual development of the Model 835 began 45 years before its recognition as the Science Heritage, in the year 1975. The time was an era when microcontrollers were being incorporated into analytical instruments with increasing frequency, and the place was Naka Works, Hitachi, Ltd. in the city of Katsuta (now Hitachinaka) in Japan’s Ibaragi Prefecture. A project leader was Dr. Shigetake Ganno, the scientist who first translated the term of HPLC as “high-speed liquid chromatography” into Japanese.
To learn more about this effort, we asked Dr. Ganno himself, who explained that it was one of the biggest projects of the 20th century—and one that would be almost unimaginable today. The best people were selected—mechanical engineers, electrical and electronic engineers, microcontroller hardware and software designers, and the sharpest technologists from Hitachi’s auditing and manufacturing divisions. Hitachi Research Laboratory also helped in developing a Japanese-made ion-exchange resin and associated analytical methods. Team members understood that their mission was to proceed organically to complete a prototype instrument in a short period of time. For example, if the development team asked the factory floor for a mechanically-processed part in the evening, a phone call would be made to a machinist who seemed likely to be able to help, and evidently he would come back to the office and deliver the requested component by 10 PM. For days on end developers would be deluged with barrages of tasks—following up on experimental results, tweaking components, rethinking planning tables, managing research expenses. Despite the demanding work environment, the team would frequently truncate their workdays to head out, en masse, for a drink or two—vowing all the while to be back at work, giving it their all, the following morning (Figure 2).
As one might expect, characterizing the performance of the instrument as a whole came down to assessing its overall performance—that is, to the analytical results it produced. A continuous sequence of analyses was performed, one after another, all day every day, in parallel with time-consuming tasks like testing component quality and lifetime and devising the countermeasures against environmental temperature. Analysis of protein hydrolysis products—the standard technique—required one hour for each test sample, allowing 24 samples per day or 72 samples from Friday to Monday; this simple observation determined the number of samples to be accommodated by the autosampler. Datacollection work to confirm the reproducibility of results continued for several months, with no break for weekends or end-of-year holidays. Finally, in the last stages of the development process—as a prototype instrument ran in a temperature-controlled chamber to test its temperature and humidity performance, and thoughts turned to publicity campaigns and advertising strategies—the team was blessed with a once-in-a-lifetime stroke of spectacular luck.

Fig. 2 The Model 835 high-speed amino acid analyzer and its developers. Counterclockwise from left: Dr. Shigetake Ganno, Mr. Yoshio Fujii, Dr. Masahito Matt Ito (an author of this article, not a developer of the Model 835), Mr. Hiroshi Satake, Mr. Akira Numata
Toward the end of April 1977, in a fishing region off the coast of New Zealand, a trawler from Japan’s Taiyo Fishery snared the remains of a distinctly reptilian creature with enormous fins, creating a stir—could this be Nessie, the Loch Ness Monster? The world was quickly abuzz with talk of dinosaurs, and paleontologists and ichthyologists gathered in Japan to investigate the possibility that the corpse might be that of a plesiosaurus. It was just after July 20, the day schools went on vacation. The only physical evidence that had been brought back to Japan from the creature sighting was a set of 42 whisker-like appendages removed by hand from the outer side of a front fin, and eventually two or three newspapers each got their hands on one or two of these precious whiskers—spurring reporters from various outlets to begin urgently requesting university laboratories to analyze the specimens.
When the Japanese newspaper Asahi Shimbun contacted Professor Tamio Yamakawa of the University of Tokyo, he introduced them to Professor Kazutomo Imahori, who knew that Dr. Ganno was in the process of developing an amino acid analyzer; eventually, this led to the whiskers being analyzed at Naka Works, Hitachi, Ltd.
Late at night on July 21, Dr. Ganno received a phone call at his home from Asahi Shimbun, who asked him to work with Koichi Suzuki, an assistant professor in Imahori’s research group, to analyze the amino acid content of the whiskers. Early the following morning, Dr. Ganno contacted Dr. Suzuki to request a survey of the published literature regarding the amino acid content of fins from various species of shark, with focus restricted to hydrolysis and to collagen sampled from fish.
The Model 835 was already set up inside a temperature-controlled chamber, and—an additional piece of good luck—Dr. Ganno had taken a holiday on Saturday the 23rd; he cut short his scheduled golf game that day and headed to the laboratory to prepare to begin analyses that evening. Throughout the afternoon, he adjusted the instrument, prepared new ninhydrin reagents, and performed test runs on standard samples. Dr. Ganno’s single greatest concern was whether the instrument’s sensitivity would suffice to allow a successful analysis; in addition to the standard method, he expected that physiological fluid analytical methods (PF method)would be necessary as well. Assuming the sample consisted of collagen, it was reasonable to expect that glycine and alanine would be abundant, and proline, hydroxyproline, lysine, and hydroxylysine should be important as well. Analysis via the standard method, including the process of column regeneration, could be performed in an hour, but PF method required two and a half hours. If he worked all night, Dr. Ganno estimated that he could complete perhaps 3 analyses.
That evening, Dr. Suzuki and an Asahi Shimbun reporter arrived at the works. To prepare samples, 23.2 mg of dried whiskers were hydrolyzed for 23 hours at 110℃ in 2 ml of 6 N hydrochloric acid, dried, and dissolved in 1 ml of 0.02 N hydrochloric acid to yield a base liquid; the scientists diluted this by a factor of 25 and attempted a test run. An atmosphere of extreme nervous tension pervaded the lab for just over 8 minutes—until the first peaks for hydroxyproline and aspartic acid appeared. The data-processing apparatus used two pen recorders operating in parallel to record chromatograms for two wavelengths: 440 and 570 nm. When the 440 nm data revealed hydroxyproline—at around 35 grids—and the peak of aspartic acid overflowed in the 570 nm data, the room breathed a collective sigh of relief. At this point, Dr. Ganno and Dr. Suzuki repeated their concentration calculations, and, as the prospects of the analysis became clear, Dr. Suzuki and the reporter decamped for their hotel.
When they returned early the next morning, the data were organized, rough quantitative calculations were performed, and a comparison to Dr. Suzuki’s compilation of published values for amino-acid content revealed that—as initially expected—the sample appeared to be taken from a shark’s fin (Figure 3).
The next day—Monday, July 25—the front page of the Asahi Shimbun reported the results in a lengthy article headlined “Principal-component proteins resemble shark fins: Mystery solved by chemical analysis of ‘whiskers,’” and the news spread quickly around the globe. As a result, the press conference at which the Model 835 was to be announced was rescheduled for the earlier date of July 28. Later that year, the Model 835 was named one of Nikkan Kogyo Shimbun’s 10 best new products of the year—capping what was, by any measure, a truly fortunate debut for the new instrument.
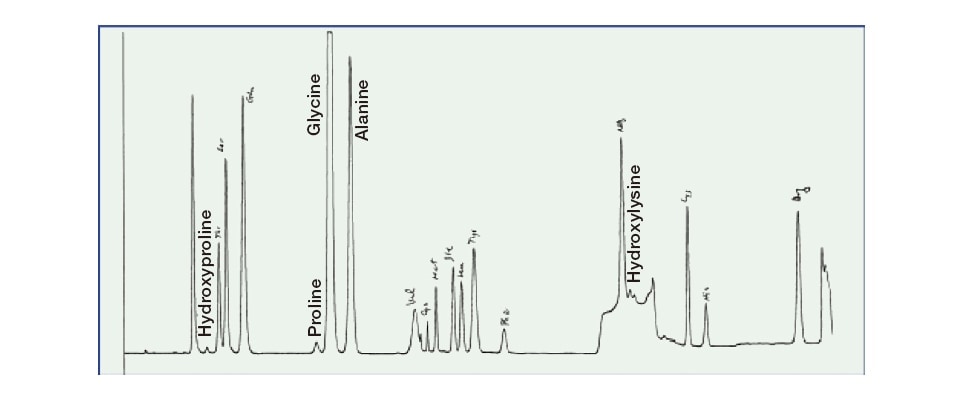
Fig. 3 Chromatogram produced by amino acid analysis of “whiskers of the dinosaur,” indicating the detection of copious quantities of hydroxyproline and hydroxylysine, substances found in collagen sampled from shark fins.
The genesis of the amino acid analyzer is said to date back to the method of ion-exchange chromatography using ninhydrin reagents, developed by Dr. Moore and Dr. Stein and announced in 19582). It was around that time that Hitachi developed two new automated analyzers based on identical operating principles, the KLA-1 and KLA-2 (Figure 4). The columns used in that era were made of glass, so the state of the packing material inside a column could be clearly seen. Because these columns were long—1.5 meters in length—they were typically operated in a vertically standing configuration to enable air to escape, essentially requiring amino acid analyzers to be rather tall. In contrast, the Model 835—although based on the same principles of separation as previous instruments—heralded the advent of so-called HPLC, as discussed below; this eliminated the need for long glass columns, allowing a floor-standing design of much shorter height than predecessors.

Fig. 4 The KLA-2 amino acid analyzer (1962). The long columns were made of glass and positioned vertically on the front instrument panel to allow their contents to be seen.
The innovation of HPLC emerged in the field of liquid chromatography in the 1970s. HPLC, a technique in which eluents were forced through columns at high pressures on the order of 10 MPa (approximately 100 atmospheres) to enable high-speed analysis, was also investigated for use in amino-acid analysis. Reducing the size of cationexchange resin particles from the conventional 40 μm to just 5 μm improved the separation performance of packing materials themselves, allowing the length of columns to be reduced while retaining acceptable separating performance. However, the transition to smaller particles reduced column permeability, which—in accordance with HPLC theory— forced a switch to stainless-steel columns and higher driving pressures. By achieving operating pressures at the 20- MPa level, the Model 835 marked an evolution from earlier generations of glass-column amino acid analyzers to a new era of high-speed amino acid analyzers based on HPLC. In passing, we note that the ultra-HPLC (UHPLC) strategy currently under development today may be interpreted as an extension of the basic HPLC paradigm—smaller particles and higher pressures—with additional desirable properties that allow increased flow velocities without sacrificing separation performance3).
The Model 835 not only reduced the physical size of the ion-exchange resin particles, but also reflected detailed research on their chemical properties. Factors such as the pH, salt concentration, and additive content of individual eluents were optimized in accordance with resin properties, and stepwise elution techniques making maximally effective use of eluents were studied in detail. This allowed analyses via standard methods to yield chromatograms sensitive even to arginine in just 50 minutes4). Equipping columns with water jackets further allowed column temperatures to be freely controlled using temperature-regulated water. These advances dramatically improved the speed and separating performance of PF method. In short, the novel ion-exchange resin and the techniques developed to exploit it were breakthroughs that lay the groundwork for HPLC.
The Model 835 reduced the inner column diameter from the KLA-2’s 9 mm to just 4 mm. The improved separation performance of the HPLC approach also reduced total eluent volumes, enabling detection of amino-acid components at much lower concentrations. (Incidentally, we note that the method of Moore et al. involved reacting with ninhydrin reagents after separating amino-acid components, and is thus classified as a post-column derivatization method5).) Reaction-chamber temperatures and other reaction conditions were also revisited in the hope of improving sensitivity, and the performance of photometers was enhanced by measures such as minimizing the flow-cell volume (Figure 5). In addition, the development of a novel pump significantly reduced detection noise and improved the baseline stability of chromatograms. Adopting the HPLC approach, and optimizing the post-column derivatization reaction system, detection system, and liquid-delivering system, allowed the Model 835 to boast a detection threshold of just 0.1 nmol— and, ultimately, to improve on the sensitivity of the KLA-2 by a factor of more than 1,0006). The ability to solve large numbers of problems like these, one after the next, reflects the comprehensive technological capabilities of a dedicated instrument manufacturer.
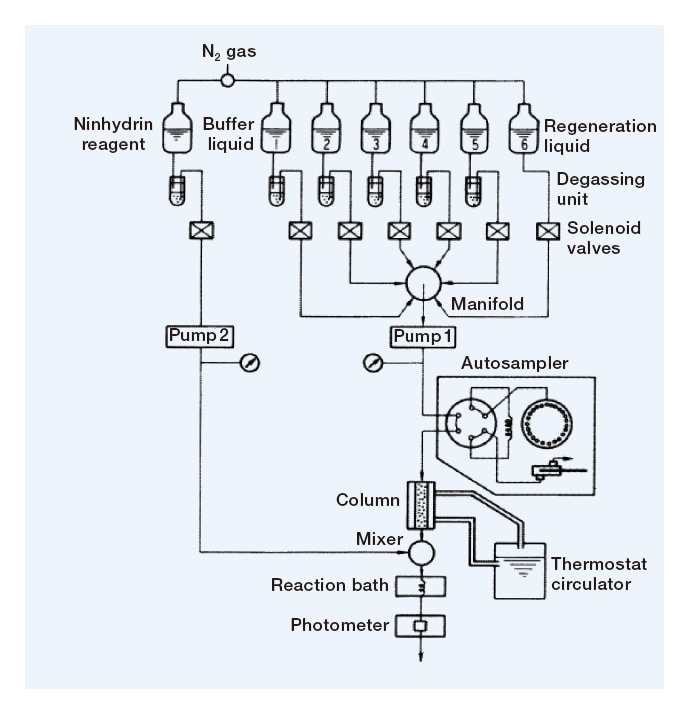
Fig. 5 Diagram of flow lines in the Model 835, whose short stainless-steel columns symbolized the arrival of the HPLC era.
When the Model 835 was developed, the microprocessor-control craze had recently begun, and everything that possibly could be automated was in the process of being automated—a trend perhaps best exemplified by the advent of the microcontroller-equipped rice cooker. The Model 835 synchronized autosampler-based sample injection with stepwise elution timing programs—including column temperature controls—and start signals for data-processor. Error/irregularity sensing capabilities for various modules—such as sensors to catch pump pressure increases—as well as column temperature regulators and other methods for detecting runaway temperatures became essential for ensuring design safety4). One truly unique feature was the introduction of magnetic storage media similar to today’s ATM cards for automated banking. Each magnetic storage card recorded an elution timing program—for an analysis by the standard method, for a PF method, etc. Users were able to execute any desired program simply by inserting the corresponding card into the card-reader slot on the Model 835’s front panel. This was a revolutionary advance at the time, and ever since there has been steady demand for operations that are simple to execute and robust against human error7).
The 835 boasted yet another key advantage: the consolidation of columns. Since the time of Moore et al., the columns used for analysis of acidic and neutral amino acids differed from the columns used to analyze basic amino acids; this was to accelerate analysis in the latter case, as basic amino acids could be separated using columns of shorter lengths than were feasible for use in acidic and neutral amino acids. The HPLC techniques introduced with the Model 835 allowed multiple distinct separation methods, which previously had required multiple distinct columns, to be carried out with just a single column. This development mindset—always targeting the cutting edge of technological possibility—has persisted all the way through to the development of today’s line of high-speed amino acid analyzers8).
The Model 835 was presented at the JASIS exhibition in September 2019, where it was received with awe by large numbers of visitors—including both veteran users of the instrument and younger researchers aware of its historic significance. One professor even opened the front panel for a nostalgic glimpse of the instrument’s internal flow lines (Figure 6)—after all, a picture is worth a thousand words! The structure and operating principles of the most recent AminoSAAYA Ⓡ instruments remain essentially those of the Model 8359). Today, for the benefit of posterity, the original Model 835 is on display for all to see in the first-floor lobby of the main building of Hitachi High-Tech’s Naka campus. The photograph of Model 835 developers in Figure 2 was taken at this location in advance of the JASIS exhibition.
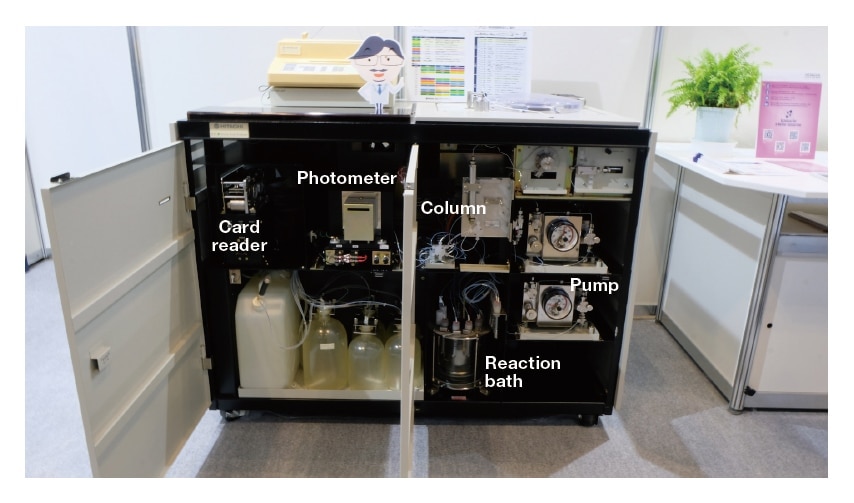
Fig. 6 Internal structure of the Model 835, photographed at the JASIS 2019 exhibition. The Model 835 was the first amino acid analyzer equipped with an embedded microcontroller.
References
1. AminoSAAYA® is a registered trademark of Hitachi High-Tech Science in Japan.
About the authors
*1. Masahito Matt Ito
Focused Solution Design Department 2
Hitachi High-Tech Science Corporation
*2. Ikuko Narimatsu, Katsutoshi Shimizu
Tokyo Science Solutions Laboratory
Hitachi High-Tech Science Corporation