8. Principle and Feature of Various Detection Methods (2)
This chapter is "Fluorescence (FL) detector","Differential refractive index (RI) detector","Conductivity detector","Electrochemical detector (ECD),"Evaporative light scattering detector (ELSD)","Corona® Charged Aerosol Detector® (Corona® CAD®)".
Fluorescence (FL) detector
A UV/UV-VIS detector monitors the absorption of light with a specified wavelength. However, some substances absorb light at one wavelength, and then emit light called fluorescence at another wavelength. This is a phenomenon in which a substance absorbs light to reach a high-energy level and then emits light to return to its original level. Such a substance has specific wavelengths of light that it absorbs (excitation wavelengths) and emits (emission wavelengths). As familiar examples, fluorescent paints and highlighters emit fluorescence with a clear color.
Figure 1 shows a FL detector optical system. While a UV/UV-VIS detector detects light that has passed through the flow cell, an FL detector detects fluorescence emitted in the direction orthogonal to the exciting light.
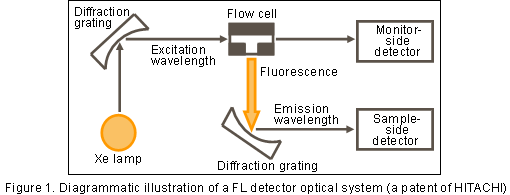
Fluorescence detection is suitable for trace analysis because of generally having high sensitivity and selectivity (not detecting impurities).
There are not many components that originally emit fluorescence (natural fluorescence). However, amino acids, etc. can be detected as fluorescent substances, after reaction with a fluorescence reagent (derivatization). This method makes it possible to measure various components with high sensitivity.
Differential refractive index (RI) detector
An RI detector detects components based on the refraction of light in solution.
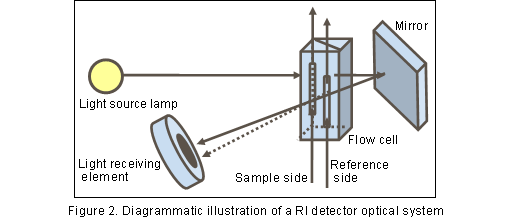
As shown in Figure2, the flow cell of an RI detector is divided into the sample-side and reference-side cells. Prior to analysis, eluate is introduced into either cell, until the flow of the eluate becomes equilibrated. The reference-side cell is filled with eluate, and the column eluate is introduced into the sample-side cell through the changed flow channel. When components are eluted from the column, the chemical composition changes in the sample-side solution, which changes its photorefractive level.
As a result, the amount of light received by the light-receiving section changes, showing a peak which can be detected. Any component in the eluate can be detected; thus, the RI detector is often called a "universal detector".
Conductivity detector
Electric conductivity measurement of a solution is a method of detecting ions in the solution.
After the targeted ions are eluted, the change in electric current is detected, with a constant voltage imposed between the electrodes. A conductivity detector is employed as a detector in an ion chromatograph, which is a system dedicated to measuring ions. This detector is used mainly to measure inorganic ions and small organic substances, including organic acids and amines.
The items shown below are often measured. Some of these items can be measured by atomic absorption analysis. However, the ability to analyze negative ions simultaneously is a particular merit of the ion chromatograph.
Negative ions; F-; Cl-, NO2-, NO3-, Br-, SO42-, PO43-
Positive ions; Na+; K+, NH4+, Mg2+, Ca2+
The conductivity detector is highly sensitive, but very susceptible to the effect of temperature variation (a change of 1℃ in solution temperature causes a change of roughly 2% in electric conductivity). Various methods of avoiding temperature variations have been devised, such as constant-temperature cells.
Suppressor and non-suppressor
In the ion chromatograph system, the electric conductivity of a solution is measured. A higher electric conductivity of an eluate produces larger noise. Two measures for this fact can be considered:
An eluent is used that originally has low conductivity.
→ Non-suppressor method
The electric conductivity of the eluate after column separation is reduced.
→ Suppressor method
The suppressor method reduces the electric conductivity by replacing Na+ with H+ or replacing SO42- with OH- in the eluate through ion exchange resins or membranes. The non-suppressor method is easy and inexpensive, because a conductivity detector is simply added to the typical LC system. Usually, the non-suppressor method can be used to measure samples with a concentration of roughly 0.1 ppm or more.
Electrochemical detector (ECD)
An ECD is used to measure components displaying oxidation-reduction reactions, and detects electric currents generated by these reactions. The ECD has high selectivity, because the necessary voltage to cause an oxidation-reduction reaction depends on the component. The ECD has also high sensitivity, and is often used for measurement of biogenic substances such as catecholamine.
Evaporative light scattering detector (ELSD)
An ELSD atomizes the column eluate, shines light on the resulting particulate components, and detects the resulting scattered light. Theoretically, an ELSD can detect any nonvolatile component. An ELSD has a sensitivity roughly 10 times higher than an RI detector, but has a slightly low sensitivity to low molecular components due to their small size. An ELSD is used mainly to detect non-UV-absorbing components. Attention should be given to the fact that nonvolatile salts cannot be used as the eluent.
Corona® Charged Aerosol Detector® (Corona® CAD®)
Like an ELSD, a Corona® CAD® atomizes the column eluate to make the sample components particulate. However, a Corona® CAD® detects them electrically by ionizing them with charged N2 gas. A merit of the Corona® CAD® is the ability to detect components with a sensitivity higher than that of an ELSD, with a sensitivity which depends only slightly on component. The eluent for a Corona® CAD® is similar to that for ELSD.
The merits of the major detectors are summarized in Table 1.
| Detector | Selectivity | Sensitivity | Merits | |
|---|---|---|---|---|
| Optical detection | UV/UV-VIS detector | 2 | 3 | A wide variety of substances can be detected that absorb light from 190 to 900 nm. Sensitivity depends strongly on the component. |
| Diode array detector (DAD, PDA) | 2 | 3 | A wide variety of substances can be detected that absorb light from 190 to 900 nm. Sensitivity depends strongly on the component. The spectrum can be confirmed for each component. | |
| Fluorescence (FL) detector | 3 | 4 | Components emitting fluorescence can be detected selectively with high sensitivity. This is often used for pre-column and post-column derivatization. | |
| Differential refractive index (RI) detector | 1 | 1 | Any component that differs in refractive index from the eluate can be detected, despite its low sensitivity. Cannot be used to perform gradient analysis. | |
| Evaporative light scattering detector (ELSD) | 1 | 2 | This detector atomizes the column eluate, and detects the scattered light of the resulting particulate components. Non-UV-absorbing components are detected with high sensitivity. | |
| Electrical detection | Conductivity detector (CD) | 2 | 3 | Ionized components are detected. This detector is used mainly for ion chromatography. |
| Electrochemical detector (ECD) | 3 | 4 | Electric currents are detected that are generated by electric oxidation-reduction reactions. Electrically active components are detected with high sensitivity. | |
| Corona® Charged Aerosol Detector® (Corona® CAD®) | 1 | 3 | This detector atomizes the column eluate and electrically detects the resulting particulate components treated with corona discharge. UV-nonabsorbing components can be detected with sensitivity higher than that of ELSD. | |
4 = Excellent, 3 = Good, 2 = Moderate, 1 = Poor
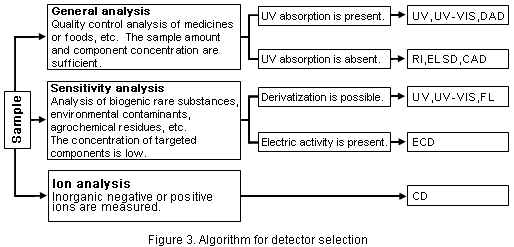
Refer to Figure 3 to select detectors.