Yuki Kamitsuma
Although titration is a classical measurement technique typically classified as a method of volumetric analysis, it is widely used as a practical method for quantitative assay measurements in quality-control analysis and research and development.
Titration measurements are typically made by using an indicator substance to detect the end point by visual inspection; however, this frequently introduces human errors caused by the experimentalist making the measurement. In contrast, automatic titrators use high-accuracy electrically-actuated burettes and end point detection based on electrochemical sensors, thus enabling high-accuracy measurements with no human errors.
The Hiranuma Automatic Titrator COM-1700A (Figure 1) is a new titrator model that retains the functionality of its popular predecessor.the COM-1700 model.while reflecting the needs of users through the addition of new features for measuring the titrant temperature and for enabling real-time display of the sample solution temperatures.
In this article, we introduce the COM-1700A with a particular focus on its newly added features.

Fig. 1 Hiranuma automatic titrator COM-1700A.
By connecting the optional titrant temperature sensor in the flow pathway between syringe and burette chip, it is now possible to measure the titrant temperature (Figure 2). The titrant temperature may be used when computing results and may also be used to correct concentrations for titrant in which the volume is susceptible to variation with temperature. (This requires reconfiguring the measurement unit separately.)
For example, perchloric acid-acetic acid standard solutions use acetic acid as a solvent; in this case, the solution volume change associated with temperature variation is large compared to titrant for which water is the solvent. When using commercial titrant, the nominal stated factors are the values at 20°C; in the past, titration had to be performed in an environment maintained at 20°C, or the factors had to be re-measured whenever the titrant temperature changed. Such tedious procedures are eliminated by the use of a titrant temperature sensor, which ensures that factors are automatically corrected.
As an illustration of the use of this feature, we here present an example involving a measurement of the purity of potassium hydrogen phthalate. For this measurement, we used a 0.1 mol/L perchloric acid-acetic acid standard solution (factor at 20°C: 1.004) and made measurements in the morning and afternoon of the same day. The measurement results are given in Table 1, while sample titration curves are shown in Figure 3. The titrant temperature differed by approximately 3°C between the morning and afternoon measurements. We find that, without factor correction, this temperature discrepancy gives rise to a discrepancy of approximately 0.3% in measured values. In contrast, performing factor correction largely eliminates the discrepancy between the two measurements. The following equations are used for factor correction.
F=F0/1+α(t-t0)
Where
F : corrected factor used for sample measurement
0 : nominal stated factor (1.004)
α : thermal expansion coefficient for the titrant (1.07 × 10-3 for acetic acid)
T : temperature at which the measurement was made
0 : temperature for which the nominal stated factor was specified (20°C)

Fig. 2 Instrument with a titrant temperature sensor.
| Time of measurement | Measurement | Titrant temperature (°C) | Sample mass (g) | Titrated volume (mL) | Purity (%) | Mean (%) | Result without temperature correction (%) |
|---|---|---|---|---|---|---|---|
| AM | Blank | 25.0 | - | 0.005 | - | - | |
| 1 | 24.9 | 0.2963 | 14.486 | 99.685 | |||
| 2 | 25.0 | 0.2943 | 14.417 | 99.873 | 99.82 | 100.36 | |
| 3 | 25.3 | 0.2984 | 14.626 | 99.897 | |||
| PM | Blank | 28.2 | - | 0.005 | - | - | |
| 1 | 28.0 | 0.2946 | 14.477 | 99.868 | |||
| 2 | 28.1 | 0.2971 | 14.598 | 99.845 | 99.81 | 100.68 | |
| 3 | 28.3 | 0.2970 | 14.577 | 99.714 |
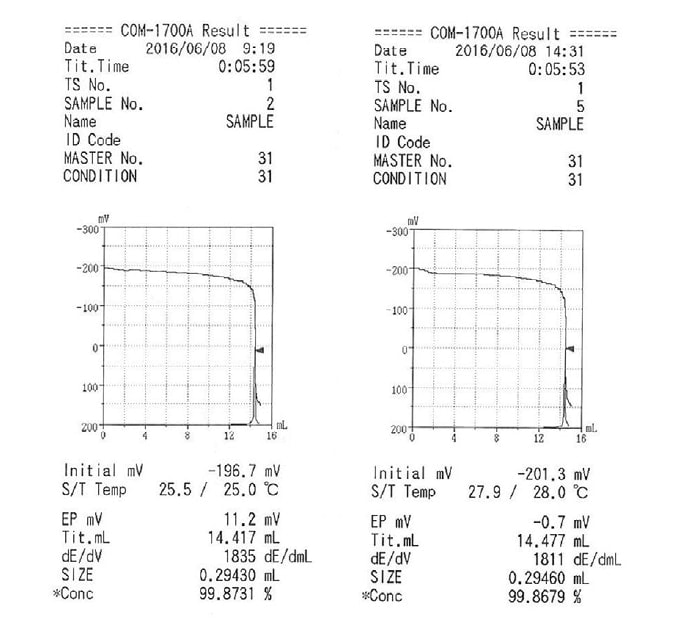
Fig. 3 Examples of titration curve printouts. (Titrant temperature: 25°C(left) and 28°C(right))
The COM-1700A is equipped with a new feature that allows real-time display of sample solution temperature within the titration cell during titration (Figure 4). This is accomplished by immersing the optional thermistor electrode (TE-403) in the titration cell. This feature is useful for the following types of titrations.
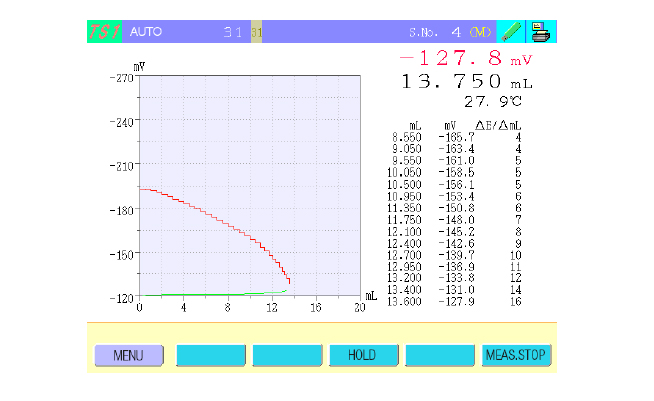
Fig. 4 Titration window.
As shown in this article, the COM-1700A is well suited for titrations that are significantly affected by temperature changes. Other features and aspects of operation are retained from the very popular COM-1700 predecessor model, ensuring that the instrument may be used in other areas as well.
Although the COM-1700A is designed as a stand alone instrument, our product lineup also includes automatic sample changer and other instrument models.
In addition, the instrument may be augmented by the addition of up to three operational units allowing up to four simultaneous measurements. We expect that the COM-1700A, with its ability to support automation of a wide variety of titration processes, will find applications in an increasingly wide range of fields.
Author
Yuki Kamitsuma
Design Department Laboratory
Hiranuma Sangyo Co., Ltd.
See more