
Otsuma Ranzan Junior and Senior High School aims to develop logical thinking and problem solving as the bases for all learning in its science education. To this end, more than one-third of its 50 chemistry basic classes each year are devoted to student experiments, to develop the students' ability to investigate through first-hand experience.
These activities are not limited to just the classroom, but also lead to the students investigating for themselves. One distinctive characteristic of the school is that students interested in scientific research choose to pursue their own self-appointed research activities after school, about which the teachers enthusiastically provide guidance. As a result, the school has won numerous awards, including the 65th Japan Student Science Award and the Minister of Education, Culture, Sports, Science and Technology Grand Award.
Today, we spoke with Misaki Fujino as she continues in her research on "palladium plating using surfactants, and its application to glucose fuel cells," and also Mr. Suzuki, under whose supervision Ms. Fujino has won numerous awards, including the Prefectural Assembly Chairman's Prize, 73rd Saitama Prefectural Science Education Promotion Exhibition. We asked them about their research activities, their first time using an electron microscope and the role it has played in their lives.

Misaki Fujino
Third-Year Student, Otsuma Ranzan Junior and Senior High School
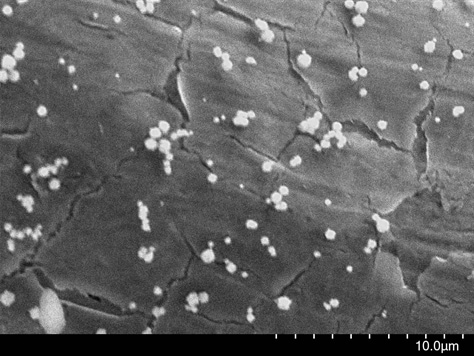
Electron micrograph of a palladium-plated surface(without surfactant)

Electron micrograph of a palladium-plated surface(with surfactant)
Suzuki:For my classes, what I value most is my students growing up and being able to tell their own children that they enjoyed chemistry class! In this sense, the electron microscope that we borrowed from Hitachi High-Tech is an amazing device that, once you use it, will change your outlook on the world and your perspective of chemistry in an instant.
Fujino:When I started high school, I didn't really have any particular interest in science. I chose calligraphy as my school club activity. The first time I found chemistry interesting was in Mr. Suzuki's class in my first year of high school. I also clearly remember the first time I used an electron microscope. In my second year of high school, I observed the compound eyes of an ant, and I became really interested in the structure of these eyes formed by orderly rows of hexagons. Being able to see this world that you normally can't see was really exciting.
Suzuki:(Smiles widely)
Fujino: It was during my second year of high school that I started to take chemistry seriously. The turning point for me was seeing my friends enjoying doing their research activities with the aim of going to conferences and competitions. I, too, wanted to participate in conferences and competitions, so I thought I'd start doing some research with this goal in mind. Since I live in an area rich in nature where I have lots of opportunities to interact with it, I asked Mr. Suzuki about me doing research on a topic to do with environmental issues and energy. He advised me to consider batteries, which led me to my current research.
Suzuki:Batteries and oxidation reduction was originally my own area of research, which is why I recommended it.
Fujino:I set out with the goal of creating fuel cells that have a lower environmental impact, so "palladium plating using surfactants, and its application to glucose fuel cells." At Otsuma Ranzan Junior and Senior High School, we've been conducting research on metal plating since 2021. Our electron microscope observations and measurements have shown that adding surfactants to a metal plating solution produces uniform and beautiful plating. So I wondered whether applying this technique to palladium plating could improve the performance of glucose fuel cells. The glucose used in these fuel cells is found in abundance in nature and is a biomass energy source. They are fuel cells that are currently in the spotlight for various reasons, including the development of implantable cells, thanks to the fact they are not harmful to living organisms.
Suzuki:While a fuel cell's resistance to degradation is an important characteristic, what gives the cell longer-lasting performance is if the palladium plating, which catalyzes the chemical reaction, is less likely to delaminate. To achieve a surface that is smoother and more resistant to delamination, we used an electron microscope to repeatedly observe plating samples using different surfactant concentration levels. We found that the surface appearance changed significantly depending on the surfactant concentration. Using the electron micrographs allowed use to right away see for ourselves what was the optimal concentration of surfactant used to create a surface with the least amount of delamination and cracking.

Teacher Takahiro Suzuki
Mr. Suzuki, who provided Ms. Fujino with guidance, himself received the Minister of Education, Culture, Sports, Science and Technology Grand Award as part of the 54th Toray Science Education Prize, which is an award to commend junior and senior high school teachers who have given creative and innovative lessons in science with good results.

Fujino presenting her research results in English
Fujino:After classes finish around 4 pm, I spend two-and-a-half to three hours doing research before I head home. I also had lots of days where I just couldn't come to any results. Like, why do I get different results when I'm doing the same thing as last time? There was also a whole two-week period where I couldn't get the electricity to flow no matter what I did. I would change the concentration, then look at the results while checking the plated surface for cracks and delamination using an electron microscope. I repeated this over and over again.
Suzuki:She really had her work cut out for her. This is the kind of task where you're almost always experiencing failure. After a year of working on it, she only had a handful of moments of making a breakthrough. It was a repetitive process with no way of predicting what would happen, or knowing if there was even the slightest chance it would work. I think she did really well to not give up.
Fujino: To check to see if a current was flowing through the fuel cell I had created, I had a motor connected to a propeller that would then rotate. After trying many, many times through trial and error, I vividly remember seeing the glint in Mr. Suzuki's eyes when the propeller finally turned.
I started this because it was something that I wanted to do, so I couldn't be the first to give up. Since I wanted to do it, I wanted to see it through to the very end.

Suzuki and Fujino using the electron microscope

Fujino is also an active member of the calligraphy club
Fujino: I finalized my research results with the aim of taking them to a competition, but in the process of doing so, I found more and more things that I don't understand. For example, during research, I found that adding carbon dioxide would improve battery performance. Placing lit objects such as incense sticks in oxygen will cause it to burn intensely. On the other hand, if you put it in carbon dioxide, the fire will go out. I hypothesized that if I added oxygen to a battery cell in the same way, then the performance would improve. And if I added carbon dioxide, the performance would worsen. But when I tested this hypothesis, I found that, to the contrary, performance improved by adding carbon dioxide. I got the same result no matter how many times I did it. But I don't understand why, and I want to understand the cause of this.
Suzuki:The battery cell we are studying uses oxygen to oxidize glucose, so it's natural to assume that if we take away oxygen then the battery cell's performance would drop. So we did an experiment to see how much performance would fall. However, the opposite of what we expected occurred. This is something that hasn't been brought up in any papers we've checked from around the world, and when I told a university professor about it, they were surprised. I think it would be amazing if we could unravel what causes this. I am currently translating her research paper into English and preparing to send it to an academic conference.
Fujino:When I think back over the past year of research, it feels like there were so many challenges. If I failed, it was a cycle of deciding to leave that feeling of dejection behind and try another approach the next day, followed by feeling dejected yet again, and so on. There were times where I found the work itself stressful, but I focused on thinking that it's just the process itself that is long, and there will be a day where I would finally see some interesting results.
I'm now in my third year of high school, and I would like to continue my research of fuel cells, if possible, at university. Someday, I hope to unravel the reasons behind how carbon dioxide improves the performance of battery cells. I hope that in the future, fuel cells will lead to research that can contribute to society in areas such as the environment and energy.


While many schools do not offer opportunities for students to do hands-on experiments in class, at Otsuma Ranzan, students do the experiments in their textbooks for themselves. In junior high school in particular, students are doing experiments practically every hour and everyone is working on scientific papers as part of their own self-directed research activities. In addition, students decide on research areas as a group, then publish their research findings as oral presentations.